03C0317

| Laufzeit: | 01.04.2001 - 31.03.2004 |
|---|---|
| Partner: |
Linde AG Engineering Division GKN Sinter Metals EUROMAT GmbH Universität Erlangen-Nürnberg, Lehrstuhl für Chemische Reaktionstechnik |
| Geldgeber: | Bundesministerium für Bildung und Forschung (BMBF) |
| Bearbeiter: | Dr. Yan Huang, Dr. Volker Höllein, Michael Jusek, Porf. Dr. Roland Dittmeyer |
| Arbeitsgruppe: | Technische Chemie |
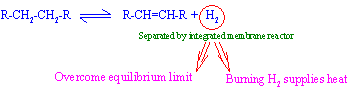
Palladium and palladium alloy membranes have been commercially used for hydrogen separators and purifiers. Interest of using them as dehydrogenation reactors is increasing.
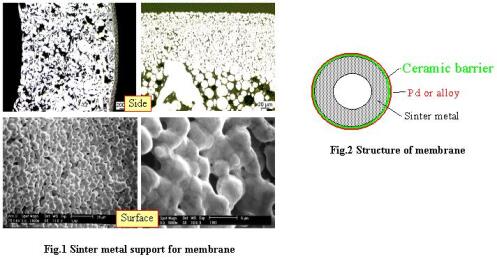
Compared with metal foil membranes, the composite membranes supported on porous substrates are promising because of the thinner thickness of palladium layer and thusly high hydrogen permeability. Porous ceramic supports were well reported for membrane plating, but one important disadvantage is the difficulty to assemble into industrial plants. Thus, sinter metal substrates were applied as membrane supports (see Fig. 1). A ceramic barrier between membrane and sinter metal supports can avoid the metal inter-diffusion during long-term operation at high temperature, and membrane plating on this barrier is it often better than plating directly over sinter metal surface. This type of membrane module is illustrated in Fig. 2. Using Pd alloy, typically Pd-Cu (40 wt%) and Pd-Ag (23 wt%), instead of pure Pd not only reduces the cost of membrane, but also allows higher hydrogen permeability and lower hydrogen embrittlement. The preparation of membranes involves two steps: ceramic barrier coating and palladium-based membrane plating.
back