
| Period: | 01.01.2019 - 31.12.2022 |
|---|---|
| Partner: | Hoppecke Batterien GmbH & Co. KG, Universität Bremen, Fraunhofer IFAM, TU Clausthal, Grillo-Werke AG, DLR Ulm |
| Associated Partner: | Accurec Recycling GmbH, be.storaged GmbH, Gaskatel GmbH, Sonnen GmbH |
| Funder: |
Federal Ministry of Education and Research (BMBF) Funding code: 03XP0204D |
| Project manager: | Willi Peters |
| Division: | Chemical Technology |
| Team: | Energy Storage and Conversion |
Background & motivation
Because of scarcity of the lithium and cobalt resources and huge growth of the accumulator-powered device market, new innovative systems have to be developed beside established Li-ion, lead-acid and NiMH technologies. The storage of renewable energy via stationary storage in batteries is still well in the hands of aqueous lead-acid and nickel-cadmium batteries. Nonetheless, LIBs with LiFePO4 cathodes (Li-LFP) are gaining new market segments due to their high energy density (up to 300 Wh/L, 130 Wh/kg) and cycling stability (> 5000 cycles). Some LFP disadvantages include flammable electrolyte and high price. Therefore, a cheap non‑flammable battery that relies on abundant recyclable materials and exhibits high cycling stability is urgently needed to satisfy increasing demand. One promising candidate is the rechargeable zinc-ion battery (ZIB).
ZIB: state-of-the-art and challenges
The zinc-ion battery is a very young technology and its working principle is similar to that of the Li-metal one (see Figure 1). During discharge step, electrochemically deposited zinc is dissolved and intercalates into the cathode matrix. When combined with a manganese oxide cathode such as α‑MnO2 [1] or δ-MnO2 [2] and a 1 M ZnSO4 electrolyte, an average cell voltage of about 1.4 V is generated and charging step can be limited to 1.85 V.
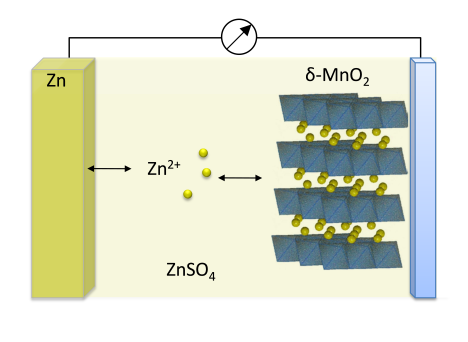 Figure 1: Working principle of a zinc-ion cell during discharging step
Figure 1: Working principle of a zinc-ion cell during discharging step
Thus, an aqueous, non-flammable electrolyte can be used. MnO2 is abundant, cheap, and environmentally friendly and its theoretical capacity of 308 mAh/g can result in a battery with an energy density close to that of Li-LFP. Recently, a cycling stability of 2000 cycles has been demonstrated by using a α‑Mn2O3 cathode [3]. However, hydrogen evolution at the end of charge, dendrite formation and long-term manganese stability are the most challenging drawbacks to overcome.
The ZIB Project
The ZIB project aims at developing both a power (200 W/kg) and an energy (80 Wh/kg) module for stationary applications, based on CuHCF [4] or MnO2 intercalation cathode materials, respectively. The tasks at DFI principally focus on material development for energy cell, and more especially for manganese-based electrodes. For this purpose, a prismatic cell will be designed in close cooperation with the battery manufacturer Hoppecke company. Additionally, material aspects related to zinc electrode, current collectors, electrolyte and separator such as e.g. hydrogen evolution, conductivity, stability, wettability and dendrite-resistance will be investigated as well.
References
[1] C. Xu, Angew. Chemi. Int. Ed. 51 (2012) 933
[2] M. Eckert, Materials 11(12) (2018) 2399
[3] B. Jiang, Electrochimica Acta 229 (2017) 422
[4] G. Kasiri, Electrochimica Acta 222 (2016) 74
backDr.-Ing. Jean-François Drillet
Tel.: +49 6172 89938-476
E-mail: jean-francois.drillet