19694 N
| Period: | 01.10.2017 - 31.03.2020 |
|---|---|
| Partner: |
Lehrstuhl für Physikalische Chemie, Universität des Saarlandes Zentrum für BrennstoffzellenTechnik GmbH (ZBT), Duisburg
|
| Funder: |
Federal Ministry for Economic Affairs and Energy (BMWi) via AiF Funding code: 19694 N |
| Project manager: | Sakthivel Mariappan |
| Research group: | Chemical Technology |
Motivation
Alarming concerns about scarcity of fossil fuels and their responsibility for air quality deterioration and global warming pushed development of cleaner and more sustainable energy carriers such as e.g. hydrogen during the last decades. In that context, Polymer Electrolyte Membrane Fuel Cell (PEMFC) is supposed to play a key role in solving challenges related to efficient energy storage and conversion. However, expansion of this emergency market still suffers from lack of hydrogen supply infrastructure, strong battery competitor, poor acceptance and exorbitant costs. Nevertheless, about 45,000 PEMFCs units were produced worldwide in 2017 that represent almost 490 MW installed power from which 90% went into transport applications; the remainder was shared by stationary CHP, prime power, off-grid and back-up systems. About 350 MW are accounted to about 3000 electric vehicles mainly produced by Toyota Company [1]. In comparison to battery-powered vehicles, Fuel Cell cars have an up to threefold larger range and can be refilled within a few minutes. Conversion of chemical energy into electricity occurs directly at platinum nano-sized particles. In one “Mirai car, “only” 15 g Pt are needed to catalyze both hydrogen and oxygen reactions and boost the 113 kW electric engine, compared to about 12 kg cobalt in a Tesla car. For shipment of 1 million electric vehicles, however, 15 tons platinum are required that represents about 8% of 2016 worldwide production. Therefore, because of geopolitical and economical reasons, long-term well-conceived strategies are indispensable. Since Pt is still considered as benchmark catalyst for PEMFC, further efforts have to be done in order to reduce electrode loading, develop recycling process and increase long-term stability. It is meanwhile well-known that alloying, temperature treatment and mesoporous carbon have a positive impact on catalyst longevity as shown in Fig. 1. Especially adequate pore size distribution is essential to confine catalyst particles and prevent their agglomeration through e.g. so-called Ostwald ripening.
Figure1: Identical location TEM images of platinum particles on (top) Vulcan carbon black and (bottom) ordered mesoporous carbon (OMC) during electrochemical accelerated degradation test (ADT) in 0.5 M H2SO4 at 1 V s-1 [2]
Objectives
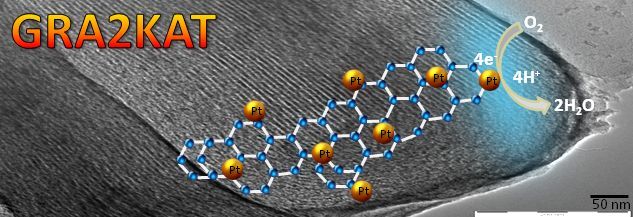
GRA2KAT project aims at the development of a middle-temperature PEMFC (MT-PEMFC) with graphenoid foam-supported Pt-based catalysts. The graphenoid samples will be produced at Saarland University and fuel cell test performed at ZBT. The DFI tasks principally focus on synthesis of mesoporous carbon from a soft-template route, development of Pt alloy catalysts, coating of gas diffusion electrodes (GDE) and finally their test under half-cell conditions.
References
[1] www.fuelcellindustryreview.com/archive/TheFuelCellIndustryReview2017
[2] M. Sakthivel, J.-F. Drillet, Applied Catalysis B: Environmental, 231 (2018), 62-72
back

Das IGF-Vorhaben Nr. 19694 N der Forschungsvereinigung DECHEMA e.V., Theodor-Heuss-Allee 25, 60486 Frankfurt am Main wurde über die AiF im Rahmen des Programms zur Förderung der industriellen Gemeinschaftsforschung (IGF) vom Bundesministerium für Wirtschaft und Energie aufgrund eines Beschlusses des Deutschen Bundestages gefördert.
Dr.-Ing. Jean-François Drillet
Tel.: +49 6172 89938-476
E-mail: jean-francois.drillet
H. Javed, S. Pani, J. Antony, M. Sakthivel and J.-F. Drillet, Soft Matter 17 (2021) 7743-7754
J. Antony, M. Sakthivel and J.-F. Drillet, ChemCatChem (2023) 15, e202201556