B. Öztürk, L. Mengis, D. Dickes, U. Glatzel, M.C. Galetz
Oxidation of Metals (2021), DOI: 10.1007/s11085-021-10088-x
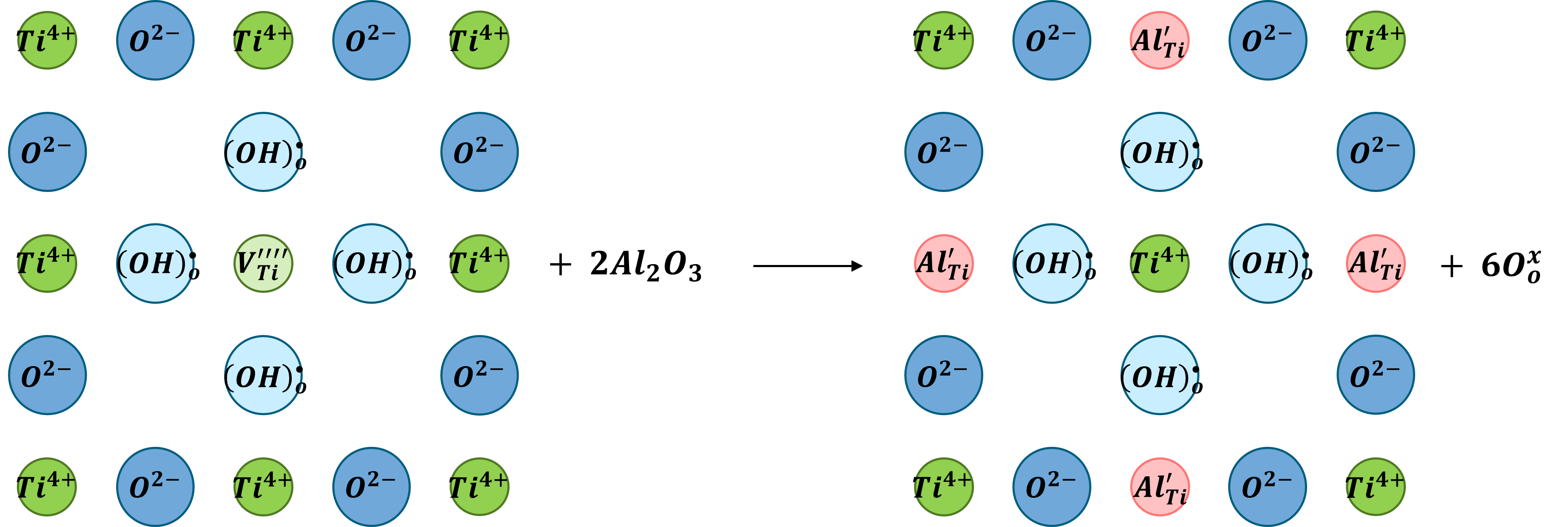
The Ti-6Al-4V alloy is extensively used in aerospace, automotive and biomaterial applications. In the aerospace industry, the service temperature of Ti-6Al-4V is currently limited to 350 °C due to its insufficient oxidation resistance. Oxidation at higher temperatures causes the formation of a fast-growing oxide scale and an oxygen-enriched subsurface layer, which is known as the “alpha-case.” Additionally, the effect of water vapor on the oxidation behavior is critical. In the present study, the oxidation behavior of Ti-6Al-4V in dry air and air containing 10 vol.% H2O at 500, 600 and 700 °C for up to 500 h has been investigated. The main focus of this study is the examination of the different oxide scale morphologies along with the oxygen enrichment in the subsurface zone. It has been observed that spallation of the oxide scale is more severe in a water vapor-containing environment. In dry air, the oxide morphology shows the typical layered TiO2/Al2O3 structure after exposure at 700 °C for 300 h, while Al2O3 precipitates are present in the outermost part of the TiO2 scale when oxidized in wet air. This indicates that the solubility and diffusivity of Al3+ ions in TiO2 are influenced by water vapor. In addition, the extent of oxygen enrichment in the subsurface zone (alpha-case) as a function of temperature and time is determined by nanoindentation profiles. It was shown that in contrast to the scale formation, the alpha-case thickness is not affected by the presence of water vapor in the atmosphere.