| Period: | 01.11.2013-31.10.2016 |
|---|---|
| Partner: | ASA Spezialenzyme |
| Funder: | BMBF |
| Project Manager: | Sebastian Bormann |
| Research Group: | Industrial Biotechnology |
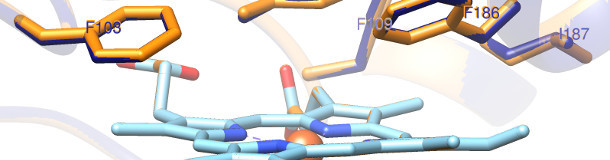
The enzyme chloroperoxidase (CPO) which is natively secreted by the fungus Caldariomyces fumago is a versatile biocatalyst with various potential industrial applications for oxyfunctionalization of organic substrates. In contrast to enzymes like p450 monooxygenases that require expensive cofactors, chloroperoxidase only requires cheap hydrogen peroxide as a cosubstrate.
Currently, low thermo- and oxidative stability prevent the industrial application of CPO. Moreover, a narrow substrate channel prevents the oxyfunctionalization of large (> C10) substrates. The aim of this project is to increase the thermo- and oxidative stability of CPO and increase the substrate spectrum by enzyme engineering.
We will develop a heterologous expression platform that will allow microtiter plate based screening of a CPO mutant library. Rational design approaches will be taken to increase the substrate spectrum of CPO. Enzyme variants with enhanced properties will be used in model reactions including supercritical carbon dioxide as an alternative solvent. Such a process addresses problems related to solubilization of hydrophobic substrates in aqueous environments. In contrast to conventional hydrophobic solvents, supercritical carbon dioxide is non-toxic, can be produced sustainably, and is easy to dispose of. The utilization of stable CPO variants in supercritical carbon dioxide processes for the oxyfunctionalization of hydrophobic substrates will thus present a sustainable alternative to chemical processes that rely on toxic and expensive solvents.
This project is carried out in cooperation with ASA Spezialenzyme. ASA is engaged in the development and production of various specialty enzymes and has extensive knowledge about formulation and utilization of chloroperoxidase.
backDr. Jonathan Bloh
Tel.: +49 6172 89938-387
E-mail: jonathan.bloh
M. Buchhaupt... J. Schrader
J Mol Microbiol Biotechnol
25:237-243