M. Lepple, S.V. Ushakov, K. Lilova, C.A. Macauley, A.N. Fernandez, C.G. Levi, A. Navrotsky
Journal of the European Ceramic Society 41 (2021), 1629-1638, DOI: 10.1016/j.jeurceramsoc.2020.10.039
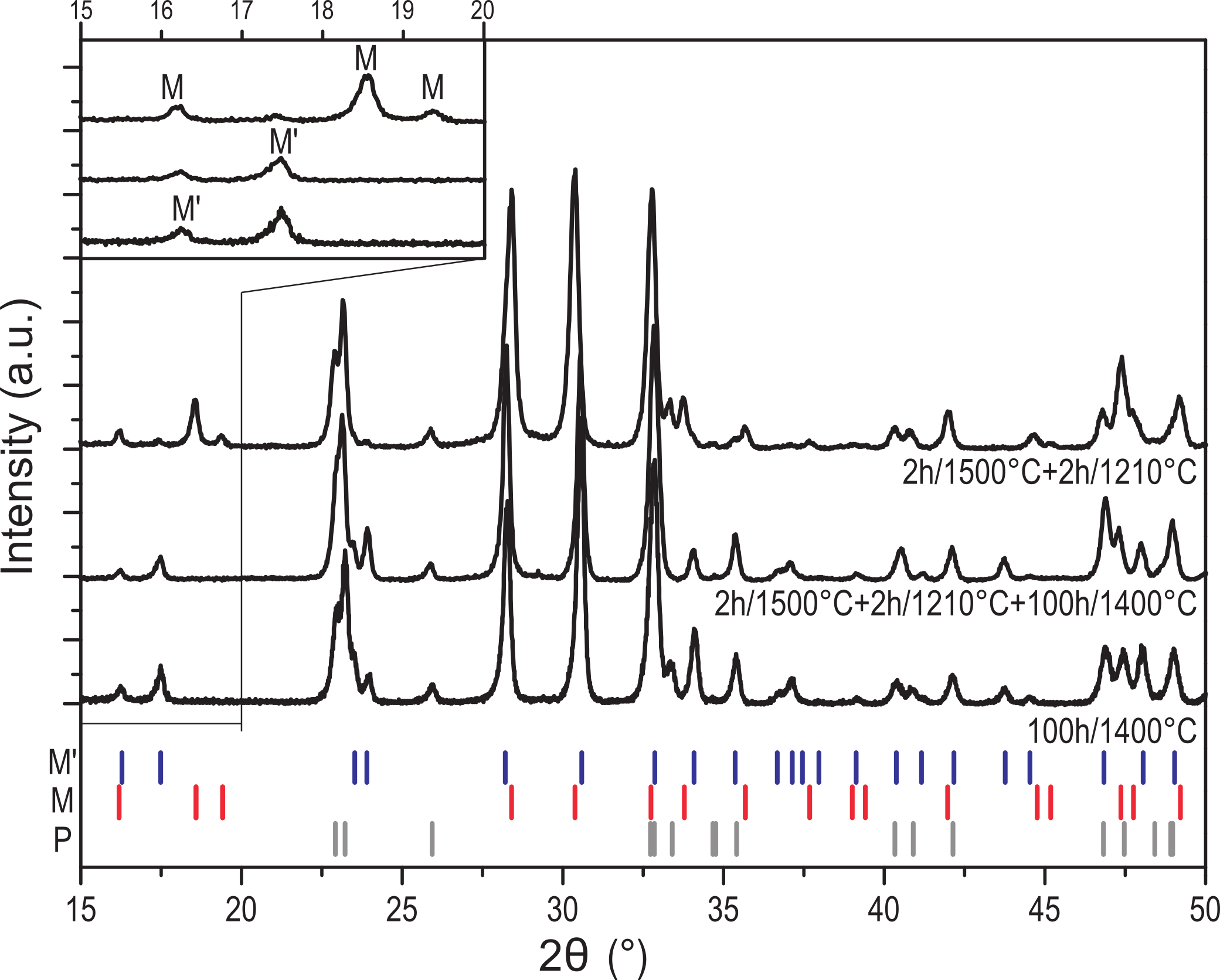
Thermodynamic parameters of the three fergusonite-related polymorphs M’, M and T of YTaO4 and their phase stabilities were investigated experimentally. The debate on the relative stability of the M and M’ phases was resolved, and it was shown that the compound does not transform to a cubic polymorph prior to melting. The enthalpies of formation of M and M’ were determined using high temperature oxide melt solution calorimetry, and the heat capacities and heat contents were measured. The enthalpy of the M’ to T phase transition was measured by differential thermal analysis. The stability of the T phase up to its melting at 2090 °C was demonstrated using high temperature X-ray diffraction and thermal analysis. Its enthalpy of fusion was determined using drop-and-catch calorimetry. The thermodynamic properties of YTaO4 assessed in this study enable the thermodynamic modeling of its polymorphs and related materials systems of technological importance.