| Period: |
01.10.2022 - 31.12.2025 |
|---|---|
| Partner: |
Karlsruher Institut für Technologie (KIT) Institut für Mikroverfahrenstechnik
Technische Hochschule Mittelhessen (THM) Institut für Bioverfahrenstechnik und Pharmazeutische Technologie |
| Funding: |
IGF (BMWK) Grant No: 01IF22612 N/1 |
| Project management: | Aneta Pashkova |
| Division: | Chemical Technology |
Motivation, aims and planned work
The application of enzymes as catalysts in chemical synthesis allows for highly selective reaction steps and therefore offers shorter and more efficient routes with less waste leading to more sustainable processes.
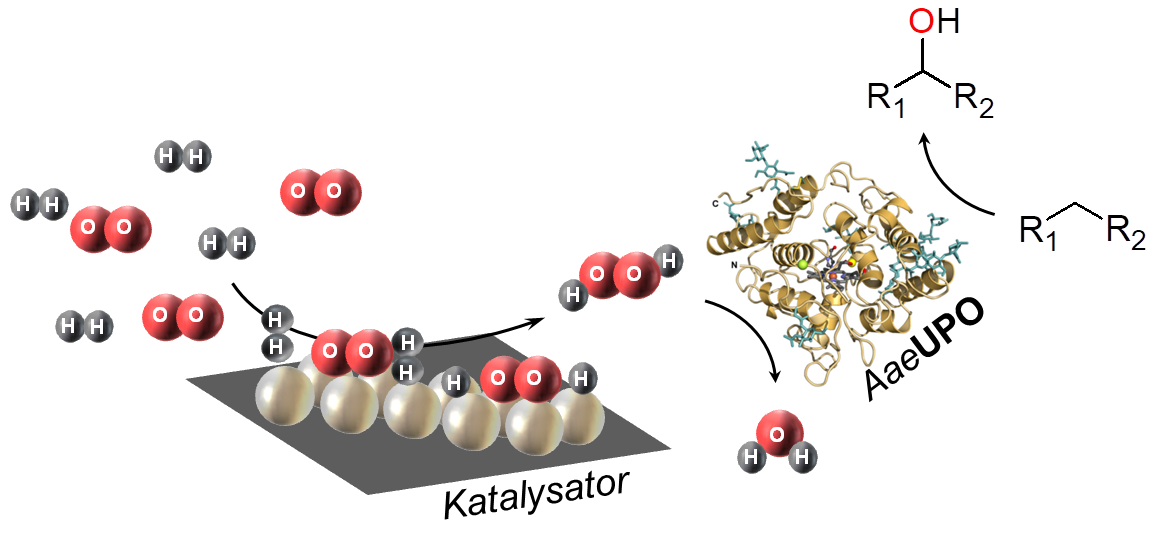
In particular, the enzyme class of oxidoreductases (EC 1) catalysing a variety of reactions are very promising for an application in organic synthesis. However, this is often limited by the need of expansive cofactors which have to be (re-)generated. Peroxygenases, like the unspecific peroxygenase from Agrocybe aegerita (AaeUPO), represent a special case as they utilize the cheap and easily producible hydrogen peroxide (H2O2) as a co-substrate to catalyse a variety of C-H-functionalizations. For example AaeUPO catalyses reactions such as the hydroxylation or halogenation of C-H bonds, the epoxidation of C=C double bonds, various heteroatom oxidations, ether cleavages, N-dealkylations and some one-electron oxidations. The enzyme also accepts a variety of different substrates.
However, a very big obstacle to the use of peroxygenases in synthetic chemistry has so far been the low stability of the enzymes towards their cosubstrate, hydrogen peroxide. On one hand, H2O2 is consumed in stoichiometric amounts for the reaction, on the other hand too high H2O2 concentrations lead to extremely rapid inactivation of the enzyme. Due to the high costs of the enzymes, the catalytic productivity of the enzymes must be significantly increased for an economic operation of a corresponding synthesis process. In order to simultaneously achieve a high turnover in the reaction and counteract the inactivation of the enzyme, the H2O2 concentration must be kept at a constantly low level, but sufficient for the reaction. The consumed amount of H2O2 must therefore either be continuously re-dosed or provided in-situ.
When dosing H2O2 or equivalent organic hydroperoxides, local hot spots and an undesired increase in volume cannot be avoided despite precise online analytics and sophisticated dosing systems. The much simpler and more elegant solution is therefore the in-situ delivery. The ways of in-situ supply of H2O2 for enzyme catalysis explored so far are: enzymatic, electrochemical or photocatalytic, each with different advantages and disadvantages. In general however, all these methods increase the complexity of the reaction and/or require specific equipment and expertise to perform.
A promising, but not yet intensively researched alternative for in-situ H2O2 supply is the chemically catalysed hydrogen peroxide direct synthesis (H2O2-DS) from the elements (H2 and O2) on heterogeneous catalysts. In contrast to the alternatives mentioned above, this approach only requires a gas supply (H2/O2) and possibly a pressure reactor, both of which are usually already available in chemical laboratories and production facilities.
Aims and planned work
The coupling of chemically catalysed H2O2-DS to enzyme catalysis will be investigated in detail and critically evaluated in this project. Here, supported noble metal catalysts will be used in conjunction with the non-specific peroxygenase from Agrocybe aegerita (AaeUPO) for the enantioselective hydroxylation of ethylbenzene. Since this model reaction has already been well described with the alternative in-situ H2O2 generation methods, an objective evaluation of the newly propsed tandem catalysis is possible. Based on the knowledge gained, a generic technical system is to be designed and optimised with regards to continuous reaction control and maximum process efficiency. In addition, the system will also be validated for further enzymatic reactions.
To enable the envisioned tandem catalysis and bring it to a technical readiness the three partners in this project will combine their individual expertise in the relevant fields. Therefore, the following work is planed:
- DFI: detailed investigation of enzyme stability under process conditions and H2O2-DS-kinetics under enzyme compatible conditions, system integration for the development of a coupled continuous system, optimization of the system and adaption to other specialty chemicals like chiral pharmaceutics or flavours
- KIT: process simulation based on the determined kinetics, design and construction of microreactors and integration of heterogeneous catalysts-through immobilization onto supports or the reactor walls via printing approaches
- THM: Development of enzyme immobilization strategies and optimization of the enzymatic productivity as well as solvent tolerance and therefore enzyme stability via molecular biological methods
back
The IGF project is funded by the Federal Ministry of Economics and Climate Protection (BMWK) within the framework of the programme for the promotion of joint industrial research (IGF) on the basis of a resolution of the German Bundestag.
Dr. Jonathan Bloh
Tel.: 069 / 75 64-387
E-Mail: jonathan.bloh
Dr. Aneta Pashkova
Tel.: 069 / 75 64-404
E-Mail: aneta.pashkova