B. O. Burek, S. Bormann, F. Hollmann, J. Z. Bloh, D. Holtmann
Green Chem., 21 (2019) 3232-3249, doi:10.1039/c9gc00633h
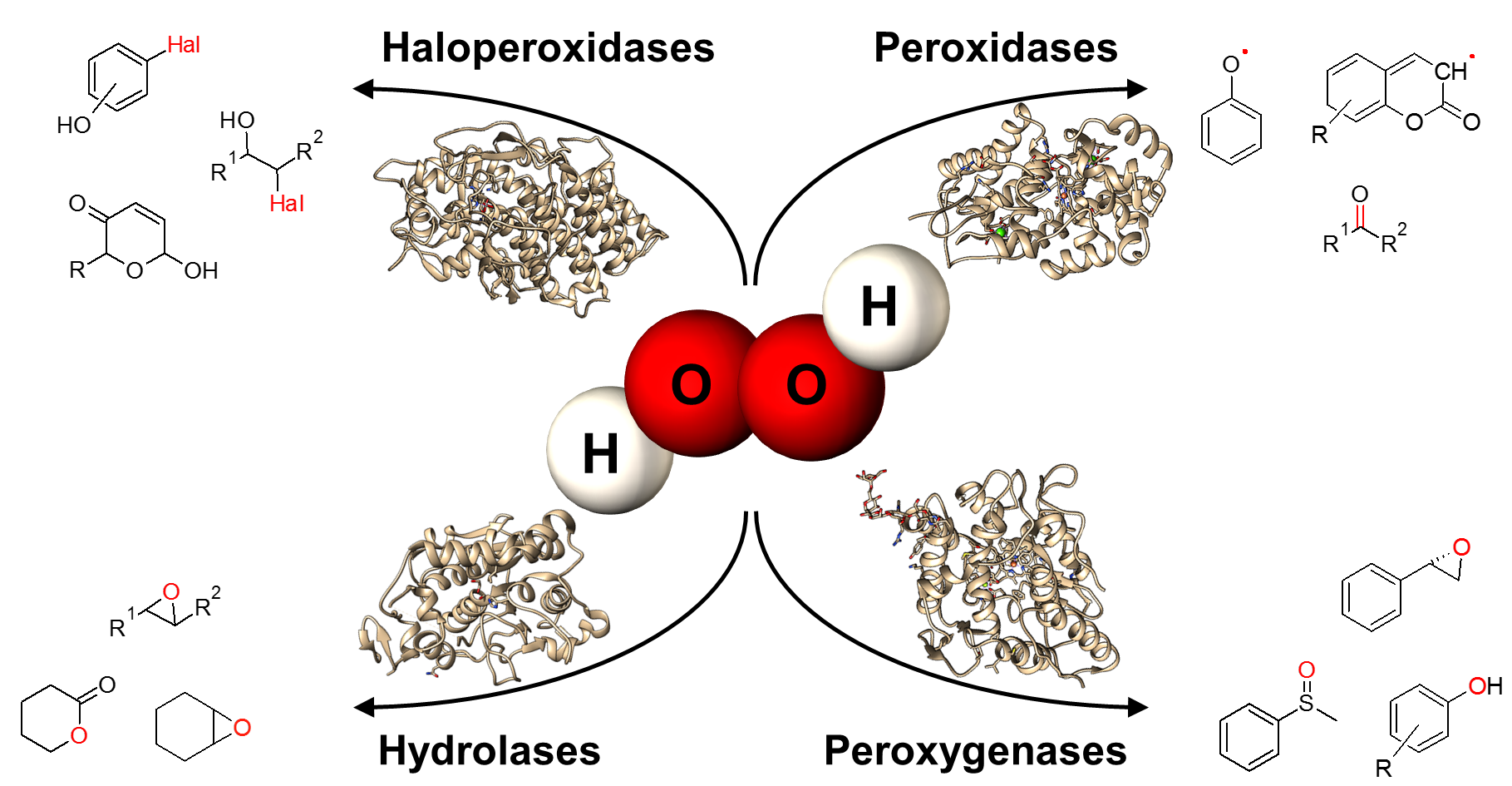
In general, hydrogen peroxide is a stable and relatively mild oxidant and it can be regarded as the ultimate “green” reagent because water and oxygen are the only by-products. Besides the direct application of H2O2 in chemical processes more and more enzymatic syntheses based on hydrogen peroxide were developed. Different types of reactions can be addressed by using a hydrogen-peroxide driven biocatalysis (e.g. hydroxylations, epoxidations, sulfoxidations, halogenations, Baeyer-Villiger oxidations, decarboxylations). H2O2-driven reactions can often be used to substitute NAD(P)H depending reactions. Therefore, laborious cofactor regeneration systems can be avoided by using H2O2-dependent enzymes. The tremendous increase in the number of publications dealing with this type of reactions clearly demonstrates the progress in this area in recent years. The described innovations range from new enzymes and types of reaction as well as novel reaction engineering approaches. This review aims to give a scope of possible advantageous applications of peroxyzymes and a critical discussion of their current limitations. The versatile reactions, the ecological advantageous and the great progress in the discovery and engineering of novel enzymes make a technical use feasible.
Bildrechte: © Royal Society of Chemistry 2019