E.M.H. White, M.-L. Bürckner, C. Schlereth, M. Bik, M.C. Galetz
Crystals 14 (2024), 60, DOI: 10.3390/cryst14010060
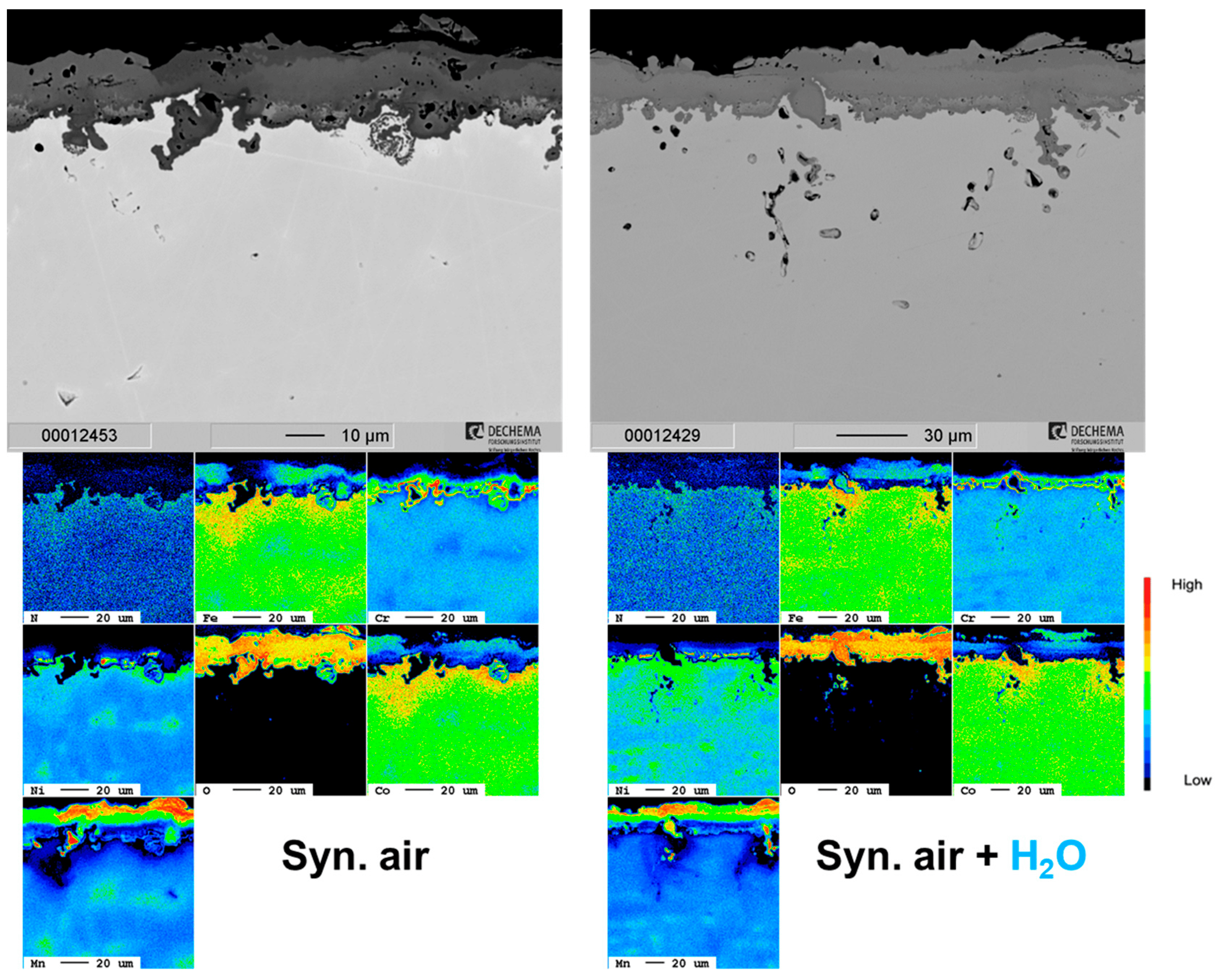
Previous studies showed some transition metal high-entropy alloy (HEA) compositions can have good oxidation resistance in air up to 800 °C. Four equiatomic HEAs have been developed based on FeCoCrNi with additions of Mn, Cu, Al or Al+Cu. The oxidation behavior of these HEAs was compared in humid (10 vol.% H2O) air at 800 °C for 100–500 h to investigate the influence of water vapor on the oxidation mechanisms. The Cu- and Al-containing alloys exhibited improved oxidation resistance over the Mn composition. For the Cu-containing alloy, a local attack of the Cu-rich phase was observed, which formed an Fe/Ni/Co/Cr spinel that was surrounded by Cr2O3. This oxide was thicker for the humid air atmosphere when compared to dry air, and the transition of the Cu oxide to the spinel was accelerated. The Al-containing HEA formed a thin Al2O3 scale with humidity suppressing AlN formation and forming a smoother oxide layer. The Al+Cu composition had the highest overall oxidation resistance (minimal local attack, no nitridation) and also showed a smooth oxide scale topography under humid air oxidation as opposed to a plate-like, rougher scale under dry air.