N.-C. Petry, A.S. Ulrich, B. Feng, E. Ionescu, M.C. Galetz, M. Lepple
Journal of the American Ceramic Society 105 (2022), 5380-5394, DOI: 10.1111/jace.18473
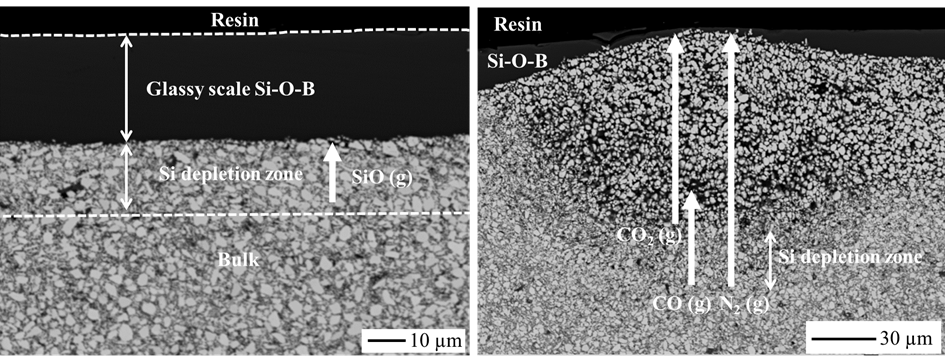
The focus of the present work is the investigation of the influence of polymer-derived ceramics, used as sintering aids for preparing ZrB2-based monoliths, on their high temperature oxidation behavior. For the preparation of the monoliths, ZrB2 powder was coated with polymer-derived SiCN, SiZrCN or SiZrBCN and subsequently densified via hot-pressing at temperatures as low as 1800°C. To investigate the oxidation kinetics, thermogravimetric analysis (TGA) was performed at 1300°C in synthetic air with exposure times of 50 h and 100 h. A detailed study of the materials oxide scale and subsurface microstructure was conducted using optical microscopy, electron probe micro analysis, scanning electron microscopy, and X-ray diffraction. The experimental findings were compared to thermodynamic equilibrium calculations using the CALPHAD method, which led to a better understanding of the oxidation mechanism. In comparison to the literature data of ZrB2-SiC, the results show improved oxidation resistance for all three investigated materials. The formation of gaseous species during oxidation, in particular CO, CO2, B2O3, and SiO, within the oxide scale of the monoliths was rationalized via CALPHAD calculations and used to explain the oxidation behavior and kinetics and also the formation of bubbles in the subsurface region of the oxidized specimens.