| Laufzeit: | 01.09.2022 – 31.08.2025 |
|---|---|
| Partner: |
VARTA Consumer Batteries GmbH & Co. KGaA (VCB) Accurec Recycling GmbH Eura AG, Branch Thuringia Gaskatel GmbH Grillo-Werke AG TU Braunschweig University of Duisburg-Essen |
| Geldgeber: |
Bundesministerium für Bildung und Forschung (BMBF) Förderkennzeichen:03XP0481B |
| Bearbeiter: | Jithin Antony, Willi Peters |
| Abteilung: | Chemische Technik |
| Team: | Energiespeicher und -wandler |
Motivation
In view of the very limited lithium and cobalt deposits and their predictable price development, it is not possible to cover the storage demand by Li-based technology alone. Here, cost-effective batteries with a long service life and abundantly available electrode materials, such as zinc and manganese, can make a significant contribution to the consumer or OEM market segment, and overarching the overall strategy. Of this, 10,300 t or 525 million are zinc-carbon (ZnC) or alkali-manganese (AlMn) cells. In addition to the classic Pb accumulators and other systems such as NiCd, NiMH and Li-ions, post-Li systems such as zinc-ion (ZIB) including zinc-manganese batteries (ZMB) with pH-neutral electrolyte could play an important role as electrochemical storage in the coming years. Compared to Li, Zn has a very high volumetric capacitance (5.8 Ah L-1 vs. 2.0 Ah L-1 for Li) relative to the negative electrode. There are no commercially available rechargeable alkaline manganese (RAM) cells on the market. Zinc Manganese battery (ZMB) is an emerging electrochemical storage (ESS) that relies on abundant, cheap, non-toxic/non-flammable electrode compounds and aqueous electrolyte.
Function principle and challenges of AA ZMB
Currently the ZIBs depends on aqueous electrolyte containing ZnSO4 with additives like Na2SO4 or K2SO4 or MnSO4 depending on the doped materials and the cathode. During the discharging step the Zn ions from the anode gets dissolved in the electrolyte and thereafter gets intercalated into the MnO2 layers. On cathode side the the Mn4+ gets reduced to Mn2+ by receiving the electrons via the circuit. As shown in Fig 1a, the Zinc ions sits in between the layers of MnO2. In subsequent charging step, the Zn ions de-intercalates from the MnO2 layer and gets deposited on the anode. On cathode side, H2 intercalation as well as the Mndeposition from the electrolyte takes place.
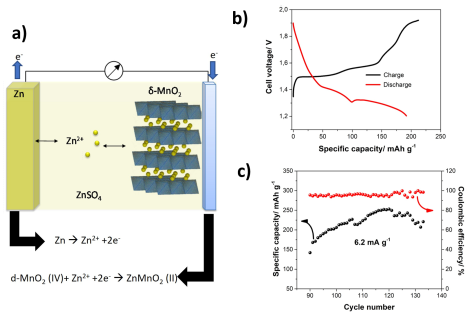
Figure 1: a) Charge-discharge mechanism in ZMBs; b) single charge/discharge step in ZMB and c) Cycling stability of ZMB with d-MnO2 on stainless sheet as cathode in 1M ZnSO4 and 0.01M MnSO4 electrolyte at 6.2 mA g MnO2-1
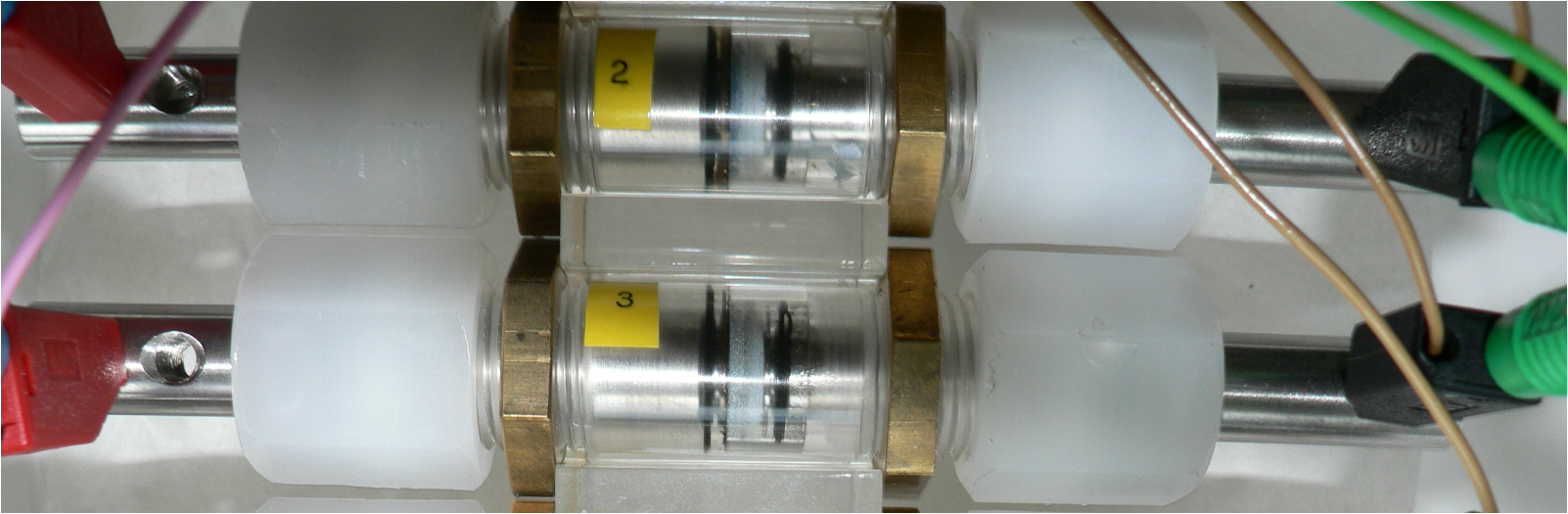
Though charge-discharge behavior of ZMBs is well-reversible for several hundred cycles in PPE laboratory cell design (see Fig. 1c), some challenging aspects related to dendrite formation, doping of the electrolyte and thinner separator material have to be overcome. While cylindrical cells offer optimal long-term protection and safe handling for customers. Since the MnO2 is less conductive and affects the performance, suitable method for the doping of MnO2 with proper elements is essential.
Project goals and work packages at DFI
DFI tasks in ZiMaBat project focus on (i) Doping and synthesis MnO2 powder and coating of the cathode. (ii) Corrosion test of passive materials (iii)Testing the materials in transparent ZMB lab scale cell. Finally, (iv) the best cell components in terms of activity, stability and corrosion will be used for the construction and test of a 1 Ah AA ZMB cylindrical cell.
zurück

BMBF Förderkennzeichen: 03XP0481B
Dr.-Ing. Jean-François Drillet
Tel.: 06172 89938-476
E-Mail: jean-francois.drillet
J. G. C. Hering, M. Holtmann, K. R. Kretschmer, J. Antony, J.-F. Drillet, D. Schröder ChemElectroChem (2025), e202400668