G5RD-CT2002-00678

| Laufzeit: | 01.05.2002 - 01.04.2005 |
|---|---|
| Partner: |
Consejo Superior De Investigaciones Cientificas, Spain
Enitecnologie S.P.A., Italy
Hermsdorfer Institut Für Technische Keramik E.V., Germany
Mast Carbon LTD, United Kingdom
Polimeri Europa SRL, Italy
Rhodia Organique, France
|
| Geldgeber: | Europäische Komission, Rahmenprogramm FP5-GROWTH |
| Bearbeiter: | Karel Svajda, Dr. Martin Reif, Prof. Dr. Roland Dittmeyer |
| Arbeitsgruppe: | Technische Chemie |
Hydrogen peroxide is an important inorganic chemical. Its use is very universal though most of the produced peroxide is consumed for bleaching of textiles and paper. The main advantage of using this particular oxidizing agent is because it is reduced to water, which is obviously an innocuous product in the waste water. Some other applications are production of peroxocompounds (peroxocarbonates, peroxoborates) used in detergents, epoxidating agent for alkenes, synthesis of caprolactam, preserving compound in food inddustry etc. Though there are a number of different routes to manufacture hydrogen peroxide nowadays it is solely produced by autooxidation of antrachinone derivatives. This procedure was implemented by I.G. Farbenindustrie at the end of World war II. The process is composed of several steps depicted bellow. At first step 2-alkylantrachinone is dissolved in a mixture of organic solvents (mostly alcohol/hydrocarbon or ester/hydrocarbon) and reduced to a corresponding chinol by hydrogen in the presence of a catalyst composed of Raney nickel and palladium on a suitable support. The suspended catalyst is then separated from the reaction mixture, and in the second step chinol derivative is oxidized in a stream of air. For this step no catalyst is required. The resulting chinone derivative is used in the first step again. The hydrogen peroxide which is formed during this step is then extracted from the solvents by water and concentrated to about 30 % aq. solution by distillation at reduced pressure.
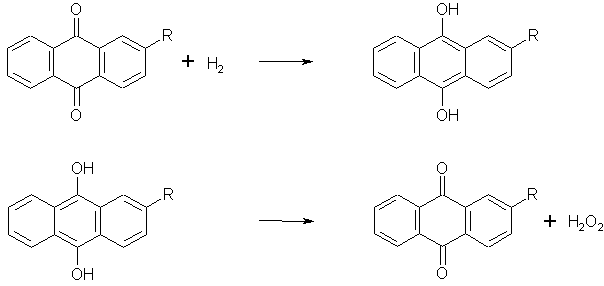
There are several side reactions that occur in hydrogenation step which increase the cost of hydrogen peroxide by consuming the chinone derivative. For this reason the economics of hydrogenation is critical for this process. It is necessary to maintain such reaction conditions under which the rates of side reactions are kept at minimum since the cost of the antrachinone derivative and solvents is about 100 times more than that of the produced peroxide.
There is a possibility to synthesize hydrogen peroxide directly from the elements. The reaction where gaseous hydrogen reacts with gaseous oxygen yielding hydrogen peroxide is highly exothermic. The reaction is catalyzed by a noble metal preferably Pd. One of the problems associated with this method is the very slow reaction rate of the formation. Also the catalyst is fairly active in decomposing the hydrogen peroxide. There have been a lot of reports about the synthesis of hydrogen peroxide with suspended catalysts. The idea of this project is to develop a two step integrated process where in the first step diluted solution of hydrogen peroxide is produced on the TCM. In the second step the diluted hydrogen peroxide solution is used for some selective oxidations e.g. propene epoxidation or benzene hydroxylation.
Our interest is to employ a membrane catalyst for this purpose. The concept is based on TCM (tubular catalytic membrane). These assymetric membranes are generally used for separation processes such as filtration. The membranes are composed of several layers having different pore sizes ranging from 3µm to 5 nm. The top membrane layer may be either Al2O3, ZrO2, TiO2 etc. In our case the membrane may be from either modified -alumina or carbon. Typical membrane is depicted below (permission of H.I.T.K.- Hermsdorf)
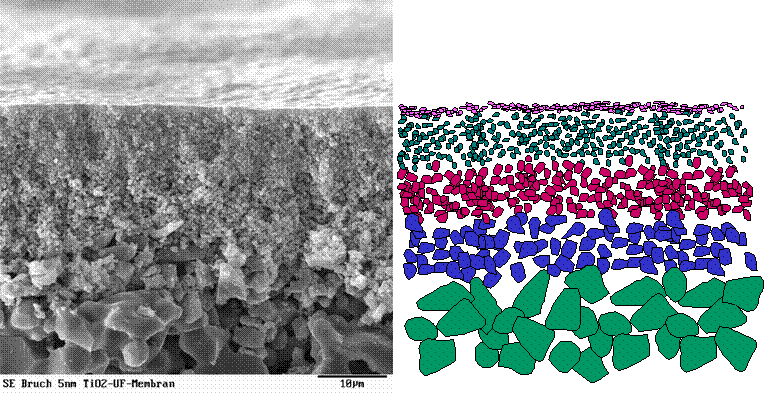
On the top membrane layer we deposit Pd by in house developed CVD (Chemical Vapour Deposition) technique. Such a membrane is then tested in the hydrogen peroxide synthesis. The experiments are performed at stainless steel pressure vessel. The performance of a membrane is dependent on various factors including radial distribution of Pd, size of its clusters, spatial arrangement, pore size of the top membrane layer etc. Several instrumental techniques such as SEM, TEM, EPMA, XRD, BET, TPD are employed to characterize the TCM.
Dr. Jonathan Bloh
Tel.: 069 / 75 64-387
E-Mail: bloh
A. Pashkova, K. Svajda, R. Dittmeyer, Chemical Engineering Journal 139 (2008) 165-171