
| Laufzeit: | 01.07.2021 - 31.12.2024 |
|---|---|
| Partner: | |
| Geldgeber: |
Bundesministerium für Bildung und Forschung (BMBF) Förderkennzeichen: 03XP0392C |
| Bearbeiter: | Charan Mukundan, Martin Eckert |
| Abteilung: | Chemische Technik |
| Team: | Energiespeicher und -wandler |
Motivation
Since market for energy storage units inexorably grow and huge demand cannot be satisfied alone by established technologies such as lead-acid, Ni-MH and Lithium-ion batteries (LIB), novel alternative concepts are urgently required. One limiting factor besides price of raw materials for widespread utilization of LIBs is related to their predicted resource shortage. While identified Li and Co reserves amount to about 54 and 7 Mio. tons, respectively, those of aluminum are estimated to reach 30 Mia.tons. By contrast, the Aluminum-ion battery (AIB) is an emerging electrochemical energy storage (ESS) technology that relies on abundant, relatively cheap, non-toxic/non-flammable electrode and electrolyte compounds. Furthermore recycling process of Aluminum is well-established and required only 10% of energy needed for production of new Aluminum.
Function principle and challenges of AIB
Current, laboratory AIBs rely on aprotic ionic liquid mixtures of AlCl3 in 1-methyl-3-ethylimidazolium chloride (EMImCl) or 1-butyl-2-methylimidazolium chloride (BMImCl) as electrolyte. During discharging step, metallic Al reacts with AlCl4– to Al2Cl7− (Al stripping) while on the cathode side, AlCl4– de-intercalate from the graphite layers as shown in Fig 1a. In subsequent charging step, Al deposition and AlCl4- intercalation occur on anode and cathode, respectively.
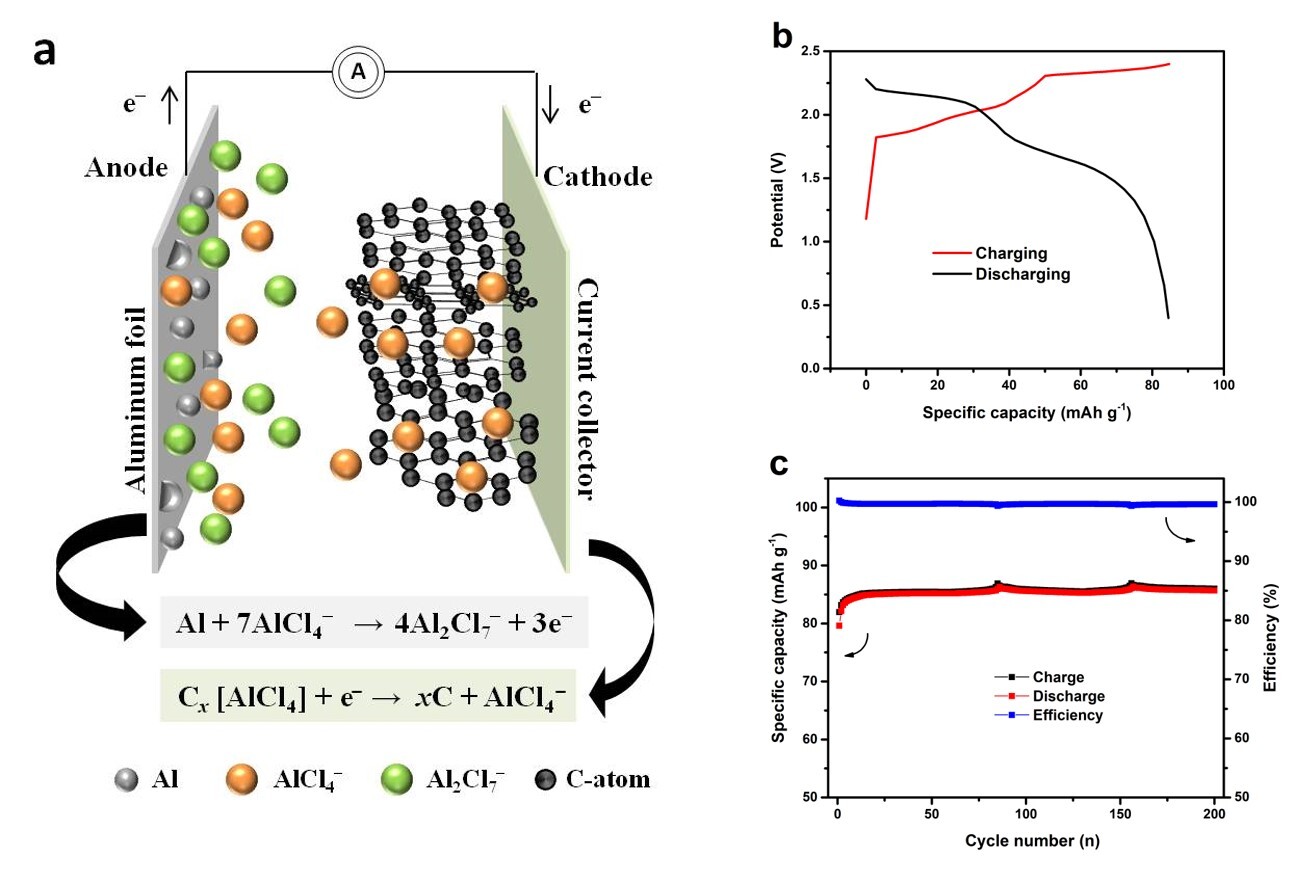 Figure 1: (a) Charge-discharge mechanism in AIBs; (b) Single charge/discharge step and (c) cycling stability of AIB with spherical graphite as cathode in AlCl3/EmImCl electrolyte at 1 A ggraphite-1
Figure 1: (a) Charge-discharge mechanism in AIBs; (b) Single charge/discharge step and (c) cycling stability of AIB with spherical graphite as cathode in AlCl3/EmImCl electrolyte at 1 A ggraphite-1
Though charge-discharge behavior of AIBs is well-reversible for several thousand cycles in PTFE laboratory cell design (see Fig. 1c), some challenging aspects related to dendrite formation, feasibility of cheaper electrolyte/electrode and thinner separator material have to be overcome. Furthermore, adequate cell designs are needed for substantial increase of energy density, especially for stationary applications. While cylindrical cells offer optimal long-term protection and safe handling for customers, attempts to use metallic casing for AIB failed due to insufficient corrosion stability of stainless steel materials in EMiMCl/AlCl3 electrolyte. Thus, search for substitution materials based on molybdenum (Mo), tungsten (W) or other adequate coatings is essential.
Project goals & work packages at DFI
DFI tasks in ALBATROS project focus on (i) evaluating kinetics parameter of Al stripping/deposition on Al alloy materials in Urea/AlCl3 electrolyte, (ii) mitigating dendrite growth into separator structure and (iii) developing a concept for protection of cell casing material against corrosion in chloroaluminate electrolyte. Finally, (iv) best materials in terms of activity, stability and costs will be selected for the construction and test of a 1 Ah 26650 cylindrical AIB cell.
zurück

BMBF Förderkennzeichen: 03XP0392C
Dr.-Ing. Jean-François Drillet
Tel.: 06172 89938-476
E-Mail: jean-francois.drillet
C. Mukundan, M. Eckert, J.-F. Drillet Batteries & Supercaps (2023), 6, e202300042
C. Mukundan, M. Lie, J.-F. Drillet Sustainable Energy & Fuels (2024)
C. Mukundan, J.-F. Drillet Batteries & Supercaps (2025)