
| Laufzeit: | 01.02.2023 - 31.01.2026 |
|---|---|
| Partner: | |
| Geldgeber: |
Bundesministerium für Bildung und Forschung (BMBF) Förderkennzeichen: 03XP0530A |
| Bearbeiter: | Meiser Valencia |
| Abteilung: | Chemische Technik |
| Team: | Energiespeicher und -wandler |
Motivation
The success of the energy transition depends on innovative and efficient electrochemical energy storage technologies. In view of the limited lithium and cobalt raw material reserve and their unpredictable price, it is very unlikely that huge upcoming energy storage demand for transportation, stationary and consumer applications with Li-based technology alone to meet net-zero emission target by 2050. Therefore, inexpensive batteries relying on abundantly available and easily recyclable electrode materials such as aluminum and graphite might significantly help storing the growing, energy produced by fluctuating, renewable energy sources. Among emerging battery systems such as Na-Ion, aprotic aluminum ion battery (AIB) could play a key role alongside established LIB, Pb & NiMH batteries in stationary segment. The main tasks of ALIBES project focus on some inherent issues related to mitigation of Aluminum dendrite growth, optimization of separator thickness and electrolyte uptake as well as improvement of long-term stability of graphite electrode. The best compounds will finally be assembled to build a cylindrical AIB cell.
Function principle and challenges of cylindrical AIB cell design
Fig. 1(a) describes the working principle and electrochemical electrode reactions during discharging step of most common AIB configuration consisting of an Al foil anode, a graphite intercalation cathode and a glass fiber separator allowing ionic transport within Lewis acidic aluminum chloride (AlCl3) based electrolyte such as 1:1.5 EMimCl-AlCl3 mixture. By contrast to Li-ion battery in which small Li+ cations (90 pm ionic radius) intercalate/deintercalated in graphite anode, AIB process, however, relies on insertion process of large AlCl4- anions (295 pm ionic radius) into graphite cathode that might lead to large extension of interlayer distance and formation of additional defects. Further objective is related to cost reduction of both ionic liquid electrolyte and Al foil.
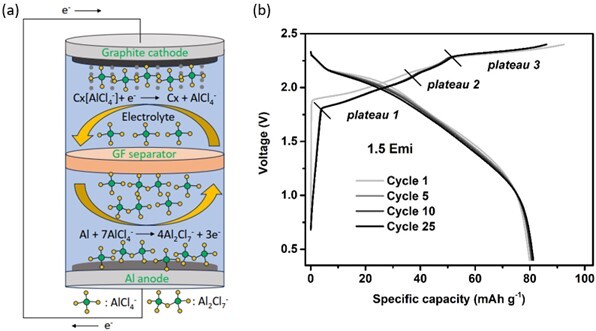 Figure 1: (a) Schematic diagram of electrode reactions during discharge step of AIB; (b) Charge-discharge curves of AIB cycled at 100 mA g-1 in 1:1.5 EMimCl:AlCl3 in a PTFE laboratory cell [1].
Figure 1: (a) Schematic diagram of electrode reactions during discharge step of AIB; (b) Charge-discharge curves of AIB cycled at 100 mA g-1 in 1:1.5 EMimCl:AlCl3 in a PTFE laboratory cell [1].
Cylindrical type cell design provides several advantages such as good mechanical resistance, excellent sealing properties, easily scalable and interchangeable cell connection as well as optimal geometry for convective air cooling. Because of high reactivity of AlCl3-based electrolyte, however, several challenges especially related to chemical stability of stainless-steel casing, Al foil anode and separator design as well as electrolyte uptake have to be solved. Fig. 1 (b) shows an example of charge-discharge curves of AIB cycled at 100 mA g-1 [1]. Three plateaus corresponding to three intercalation stages of AlCl4- ions into graphite structure are observed during the charging process.
Project goals & work packages at DFI
The aim of this project is to demonstrate the feasibility of a rechargeable 1 Ah aluminum-ion battery with at least 100 charge/discharge cycles in the cylindrical 32650 format towards the end of the project. The main tasks of DFI include: (i) development of a corrosion-resistant 32650 cell housing; (ii) test of the cylindrical AIB cells with basic components; (iii) investigations with different electrolyte purities; (iv) test of the AIB cylindrical cell with advanced components.
Reference
zurück

BMBF Förderkennzeichen: 03XP0530A
Dr.-Ing. Jean-François Drillet
Tel.: 06172 89938-476
E-Mail: jean-francois.drillet
Dr. Meiser Valencia
Tel.: 06172 89938-415
E-Mail: meiser.valencia