P. Hutterer, M. Lepple
Journal of the American Ceramic Society (2022), DOI: 10.1111/jace.18832
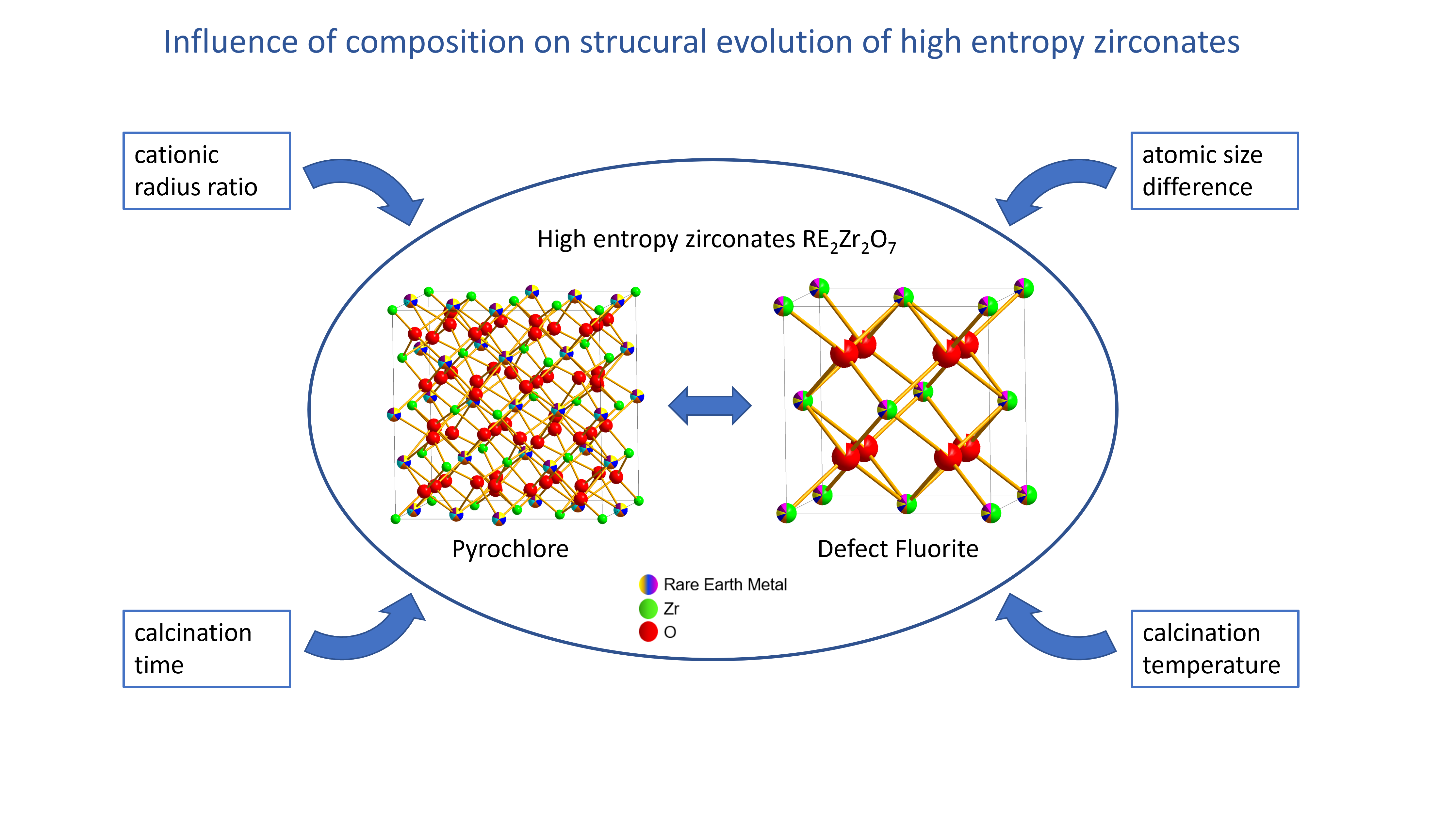
Ten different high-entropy rare-earth zirconates with the general formula of A2Zr2O7 were synthesized using reverse coprecipitation. Thereby, five cations were mixed on the A sublattice, and their composition was varied systematically regarding cation size to vary the cationic radius ratio rA/rB and the atomic size difference δA. Phase and chemical composition as well as morphology of the synthesized materials were examined by X-ray diffraction (XRD), scanning electron microscopy, energy-dispersive X-ray spectroscopy, electron backscatter diffraction, and electron probe microanalysis. Additionally, their phase stability was investigated using high-temperature XRD and differential scanning calorimetry. Single-phase materials were obtained when δA was below 4.5%. This threshold value was determined and verified using additional data taken from literature. The single-phase compositions formed pyrochlore or defect fluorite structure depending on their rA/rB with a threshold value of 1.46 being the same as for binary zirconates. Furthermore, the single-phase compositions remained stable up to high temperatures.