| Period: | 01.04.2021 – 31.12.2025 |
|---|---|
| Partners: |
Deutsches Zentrum für Luft- und Raumfahrt (DLR) Europäisches Institut für Energieforschung (EIFER) Fraunhofer-Institut für Produktionstechnik und Automatisierung (IPA) Zentrum für Brennstoffzellen Technik (ZBT) |
| Funding: |
Federal Ministry of Education and Research (BMBF) Grant No: 03HY110A |
| Project management: | Dr. Nicky Bogolowski, David Kniep, Dr. Sakthivel Mariappan, Beatris Sanchez Batalla |
| Teams: | Applied Electrochemistry, Energy Storage and Conversion, High Temperature Materials |
Background
Through decentralized electrolysis of water from renewable energies like photovoltaics or wind power, hydrogen can be obtained as a chemical energy storage, which then can be used as an important raw material in the chemical industry e.g., for steel or methane production. On the one hand, this leads to a reduction in CO2 emissions in these processes, and on the other hand, the conversion to sustainable sources of raw materials is being promoted. For large-scale implementation of the production of hydrogen via renewable energies, however, large capacities of high-performance, cost-effective electrolysers are required.
This is where the hydrogen flagship project H2Giga, initiated by the Federal Ministry of Education and Research (BMBF) of Germany, comes into play by preparing and advancing the industrialization of water electrolysis for production of green hydrogen. Amongst others, the production technologies required for production of electrolysers e.g., automation, digitization and methods for quality control are to be developed, converting manual production with a low level of automation to industrial series production for the corresponding market ramp-up.
PEMEL, AEL und HTEL Technology
In technical water electrolysis, three different processes are relevant for hydrogen production. In PEM electrolysis (PEMEL) with proton-conducting polymer electrolyte membrane, the gas hydrogen is obtained from liquid water and electric current on the cathode side while oxygen evolves on the anode side. In contrast, alkaline electrolysis (AEL) uses basic electrolytes such as potassium hydroxide solution and hydroxide ion-conducting diaphragms. Both technologies are carried out at temperatures up to 100°C. The high-temperature electrolysis (HTEL) of water vapor takes place at temperatures between 700 and 900°C. Due to the temperature, oxygen-ion-conducting ceramic electrolytes are used here. The technology is like that of high-temperature fuel cells (SOFC).
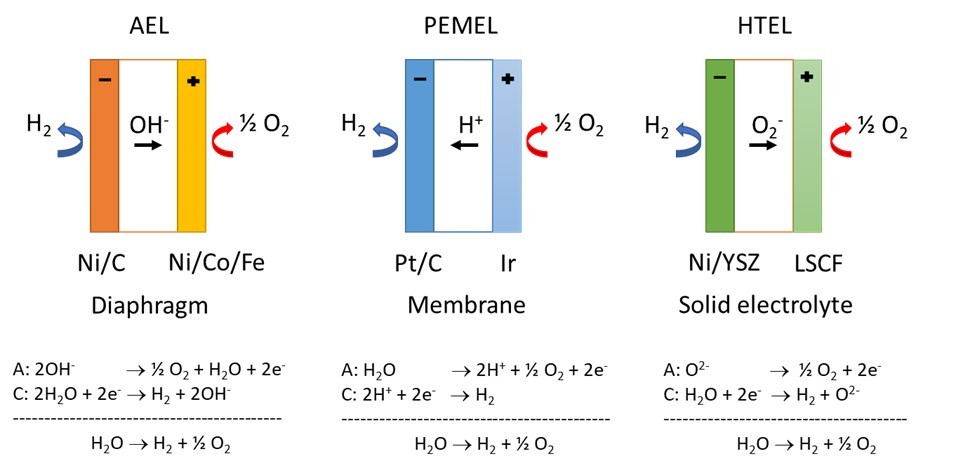
Alkaline water electrolysis is the most technically advanced technology for the industrial production of hydrogen. Conventional AEL use low-cost, nickel-based electrodes with a long life time, but only low operating current densities and stack efficiencies can be achieved. PEM electrolysis is becoming increasingly important. However, since catalysts containing precious metals such as Pt or Ir are used, investment costs are significantly higher compared to alkaline electrolysis. High-temperature electrolysis is characterized by a higher degree of efficiency but is currently even less technologically mature.
Objectives and DFI tasks
Main objectives of the Degrad-EL3 project in H2Giga are the identification of degradation mechanisms of the components (electrodes, membrane/electrolyte, bipolar plates, interconnectors, peripherals) of PEMEL, AEL and HTEL technology and the development of methods for predicting the lifetime of the various systems.
Three DFI teams are working on the degradation mechanisms of all three electrolysis technologies (AEL, PEMEL & HTEL). With the help of three newly acquired test setups, the service lifetime and degradation cause of single cells and short stacks up to 1 kW are to be estimated or identified using accelerated stress procedures and endurance tests of up to 1000 h. The influence of different operating states, such as start & stop procedures, load changes, constant load or open-circuit operation (OCV) on the respective electrolyser performance is determined. Changes to parameters such as cell/stack voltage, impedances, energy efficiency as well as H2 or O2 selectivity are intended to provide valuable information about the kinetics of the respective degradation processes. After end of experiment, the relevant components of the different electrolyser will be examined through post-test analysis, such as XRD, SEM/EDX/WDX, Raman spectroscopy and XPS, and typical degradation phenomena be identified in more detail. The corrosion resistance of the individual components will also be evaluated by using accelerated chemical degradation tests.
back

BMBF Grant No: 03HY110A
Dr.-Ing. Jean-François Drillet
Tel.: 069 / 75 64-476
E-Mail: drillet
Dr. Nicky Bogolowski
Tel.: 069 / 75 64-177
E-Mail: bogolowski
D. Kniep, S. Schewe, M. Rudolphi, M.C. Galetz, International Journal of Hydrogen Energy (2024)
M. Sakthivel, J.-F. Drillet, ECS Transactions (2024) 114/5
V. Trouche (Adele Hydrogen), H. Doan (Adele Hydrogen), N. Bogolowski, J.-F. DrilletAdele Hydrogen Technology Insight #1 (2025)