DR 812/1-1

| Period: | 01.05.2010 - 30.04.2013 |
|---|---|
| Partner: | Max-Planck-Institute of Carbon Research, Mülheim (Ruhr) |
| Funder: | Deutsche Forschungsgemeinschaft (DFG) |
| Project Manager: | Dr. Sakthivel Mariappan, Dr. Jean-François Drillet |
| Research Group: | Chemical Technology |
Fuel cells are usually classified into working temperature categories. High temperature fuel cells (HTFC), such as the Solid Oxide Fuel Cell (SOFC) or the Molten Carbonate Fuel Cell (MCFC) are working in a temperature range of 600-950°C that allows a sufficient conductivity of the electrolyte. State of the art HTFCs have already shown high cell efficiency up to 60%. Low temperature fuel cells (LTFC) are mostly equipped with a polymer membrane such as Nafion whose conductivity depends on the presence of water molecules. Therefore, their working temperatures are usually limited to 80-90°C. With exception of MCFC that is specially designed for stationary electricity plans, both, high and low temperature fuel cells are planned to be used in a foreseeable future as energy converter for stationary and automotive applications. In the case of the LTFC, however, more robust systems and especially, more stable polymer membranes than PBI-based ones, which are still sensitive to cold starting processes that are able to work at 100-150°C are needed. Higher working temperatures mean higher efficiency of the catalysts, lower electrolyte resistances and as a consequence higher cell performances. These depend not only on the working temperature, kind of catalyst and membrane, but also on the purity of the fuel and its distribution within the diffusion and reaction layers and also on the evacuation of the reaction products, which can lead to catalyst poisoning and electrode flooding, respectively. The latter depends on the morphology and properties inherent to the diffusion and reaction layers, such as catalyst loading, porosity, hydrophobicity, thickness and additionally on the compression forces within the stack. For these reasons, the design of the membrane-electrodes assembly (MEA) remains a very important step within the fuel cell concept. One distinguishes two strategies: the most common one consists on coating the electrodes with the diffusion and reaction layers (CCE) and finally press them together with the membrane to a MEA. The second one aims to directly coat the membrane with the reaction and diffusion layer inks or pastes (CCM).
Ten German research institutes with complementary competences are involved in a AiF/DFG- cluster project that aims at the development of a middle temperature polymer fuel cell (MT-PEMFC). The list of these institutes as well as the cluster structure are shown in figure 1. This cluster includes four subprojects (TP). The first one (TP1) aims at the development of novel polymer membranes that are able to work in the temperature range of 100-150°C. The second one (TP2) focuses on the preparation of more efficient carbon supported Pt and Pt bimetal catalysts for the oxygen reduction in the hydrogen (H2-PEMFC) and methanol (DMFC) fuel cells (TP2). Different coating techniques such as screen-printing, spraying, sputtering and galvanic deposition onto either the gas diffusion layer (GDL) or the membrane will be improved and compared with each others in TP3. The ultimate goal in TP4 is the construction of two stacks with working temperatures in the range of 100-150°C:
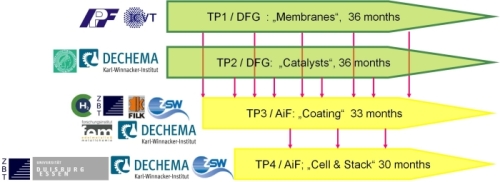
Leibniz-Institut für Polymerforschung, Dresden (IPF)
Institut für Chemische Verfahrenstechnik, Universität Stuttgart, (ICVT)
Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr (MPI)
DECHEMA, Karl-Winnacker-Institut, Frankfurt am Main (KWI)
Hydrogen Institute of Applied Technologies, Schwerin (HIAT-H2)
Zentrum für BrennstoffzellenTechnik GmbH, Duisburg (ZBT)
Forschungsinstitut für Leder und Kunststoffbahnen, Freiberg (FILK)
Zentrum für Sonnenenergie- u. Wasserstoff-Forschung, Ulm (ZSW)
Forschungsinstitut für Edelmetalle und Metallchemie, Schwäbisch Gmünd (FEM) Energietechnik, Universität Duisburg-Essen, Duisburg (UDE)
Figure 1: Organisation of the cluster with interconnection of the different TPs for material delivery timing and list of the involved institutes.
This project aims at the development of highly active carbon-supported bimetal Pt catalysts for the ORR in the middle temperature (100-150°C) fuel cell cathode (H2-PEMFC and DMFC). Despite the large and growing interest of polymer electrolyte membrane fuel cell (PEMFC) as an energy converter, high cost, relatively insufficient activity and long-term stability of the catalyst materials remain major obstacles an extensive commercialization. Platinum is still considered as the state-of-art for catalyst material in PEMFCs. However, it exhibits a slow kinetics for oxygen reduction reaction (ORR) [1] that is mostly due to the strong bond of the adsorbed oxygen molecules on the surface. Meanwhile, bi-metal catalyst such as PtNi, PtCo, PtFe have shown higher activity for ORR compared to Pt also in presence of methanol [2] The incorporation of transition metal alters the electronic d-band structure of Pt (ligand effect) and the Pt-Pt bond distance (compressive/tensile strain) that in some cases leads to a decrease of adsorption energy of oxygen molecule and consequently to higher activity for ORR. The ORR activity of the Pt alloy depends also on the type and concentration of the second metal in the subsurface atomic layer [3]. A suitable bi-metal alloy should favor the "four electron" reduction step of oxygen as shown in the eq. (1). In eq. (2), H2O2 intermediates step is involved that is unfavorable for the catalyst efficiency and chemical stability of the polymer membrane .
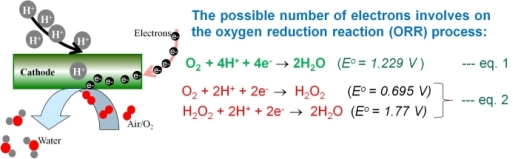
Figure 2: Illustration of the oxygen reduction reaction at platinum
KWI: • Synthesis of high active Pt based bimetal alloys with early and late transition metal elements (Ni, Cr, Co, Ti, V…) • Optimization of carbon supported catalyst structure, particle size, dispersion, composition, stability through physicochemical characterization • Investigation of new catalyst architecture (Core-Shell) • Activity evaluation for ORR with electrochemical methods using rotating (ring) disk electrode (RDE/RRDE) under half-cell conditions • Fabrication of gas diffusion electrodes (GDE) and characterization with current-voltage polarization and impedance spectroscopy in GDE cell. • Evaluation of particle size and alloying level with XRD and TEM.
MPI: • Synthesis of high graphitized carbon supports (hollow spheres) • Encapsulation of the Pt and PtM catalyst within the hallow spheres • Characterization of their physical shape, size, structure, metal loading and chemical compositions with HRTEM, TGA and XPS.
Literature
[1] Y. Bing, H. Liu, L. Zhang, D. Ghosh, J. Zhang, Chem. Soc. Rev. 39 (2010) 2184.
[2] S. Mukerjee, S. Srinivasan, J. Electroanal. Chem. 357 (1993) 201.
[3] V.R. Stamenkovic, B.S. Mun, M. Arenz, K.J.J. Mayrhofer, C.A. Lucas, G. Wang, P.N. Ross, N.M. Markovic, Nat. Mater. 6 (2007) 241.
Dr.-Ing. Jean-François Drillet
Tel.: +49 6172 89938-476
E-mail: jean-francois.drillet
M. Sakthivel ... J.-F. Drillet, J. Electrochem. Soc., 162/8 (2015), F901-F906
M. Sakthivel ... J.-F. Drillet, ECS Trans., 61/31 (2014), 15-24
M. Sakthivel, J.-F. Drillet, Electrochimica Acta, 120 (2014), 73-79