| Period: | 01.01.2018 – 30.06.2021 |
|---|---|
| Partner: | IoLiTec GmbH, Fraunhofer ILSB, Fraunhofer IZM, TU Clausthal, RWTH Aachen |
| Funder: |
Federal Ministry of Education and Research Funding code: 03XP0128D |
| Project manager: | Martin Eckert |
| Divison: | Chemical Technology |
| Team: | Energy Storage and Conversion |
Motivation
Lithium – ion batteries (LIB) are actually the most common used battery technology for mobile applications (e.g. smartphones, notebooks, tablets)[1]. It is probable that classical battery systems such as electrically rechargeable alkaline NiMH used in flashlights or remote controls will be replaced also by LIBs in the future. Additionally, LIBs are actually the most promising storage technique for being used in electromobility (e.g. Tesla). Relatively low energy densities, limited raw material resources and rapidly increasing prices (especially of cobalt) and security aspects are the most significant limitations of this technology. Therefore, development of post-lithium energy storage technology is urgent. Aluminum has a four times higher volumetric energy density than lithium (8.04 Ah/cm³ vs. 2.08 Ah/cm³) and its concentration amounts approx. 7.5 % in the earth’s crust. This makes Al to an extremely attractive material for a sustainable battery technology.
State-of-the-art and challenges
One of the most important challenges for developing a secondary aluminum battery consists on finding an electrolyte which both allows reversible Al dissolution/deposition at the anode and Al-ion intercalation/de-intercalation at the cathode. Since Al deposition/dissolution occurs at very negative redox potential (-1.67 V vs. NHE), charging reaction step is not possible in aqueous solutions because of hydrogen evolution. Also formation of passive aluminum oxide layer in presence of residual water is thermodynamically favorable and can be dissolved in chloride-containing electrolyte. Ionic liquid mixtures like 1-ethyl-3-methylimidazoliumchloride (EMIMCl) / AlCl3 are able to dissolve and deposit Al reversibly because of formation of the active aluminum-ion species Al2Cl7-.
Recently, an ultrafast rechargeable aluminum battery was presented by Lin et al.[2] in which pyrolitic graphite foam was used as cathode material, EMIMCl / AlCl3 as electrolyte and pure Al metal as anode. This cell had a coulombic efficiency of ~98 % over 7,000 cycles at very high current densities. Angell et. al.[3] reported on a similar cell using same electrode materials but using an less aggressive urea/AlCl3 deep eutectic solvent mixture. This cell had a coulombic efficiency of 99.7 % over 200 cycles. The use of graphite as cathode material however implies intercalation of large AlCl4- anions and strongly limits cell capacity values up to about 70 – 150 mAh/gcathode[1,5]. Therefore oxide-based Al insertion materials are desired. Brown et. al.[4] published a patent in 2012, where an aluminum – ion battery with a manganese oxide spinel (Mn2O4) cathode should provide extraordinary high theoretical capacity and energy density (400 mAh/g and 1,060 Wh/kg). Fig. 1 shows the two different cell designs during discharging step, where the left one is based on anion intercalation and the right one on cation intercalation such as in typical LIB systems. The theoretical maximal capacity of MnO2 is ~308 mAh/g. One of the most challenging topics deals in that case with dissociation of positive aluminum ions at electrode/electrolyte interface.
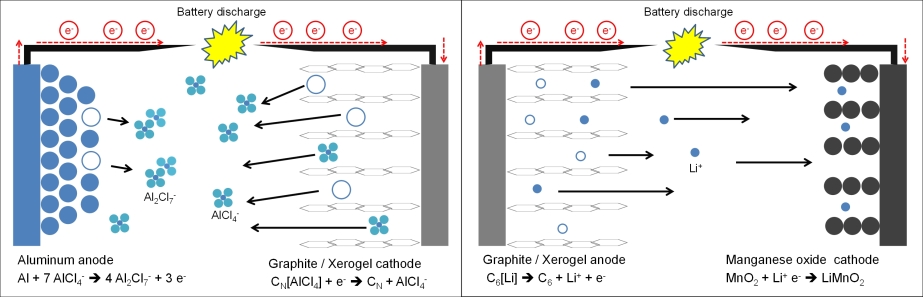
Fig. 1 Left: Cell composition published by Lin et al.[1]: Aluminum as anode, graphite as cathode and a mixture of EMIMCl / AlCl3 as electrolyte. Right: Typical cell composition of a LIB battery with MnO2 as cathode, graphite as anode and a lithium containing electrolyte (e.g. LiPF6). The ⃝ marked locations belong to former positions of ionic species (AlCl4- or Li+) or metallic aluminum.
Aim of AliBatt project
The main objective is to develop an aluminum-ion battery with high energy density for mobile applications as alternative to LIBs. The primary tasks at DFI are related to development of performing intercalation cathode materials e.g. highly porous graphite, cheap aluminum alloys for the anode and effective electrolytes. Half-cell and full-cell measurements should provide precious information about the electrochemical behavior of these components.
Literature
[1] R. Korthauer, Handbuch Lithium-Ionen-Batterien, Springer Verlag Berlin Heidelberg, 2013
[2] M. C. Lin et. al., Nature 520, 2015, p. 324 - 328
[3] M. Angell et. al., PNAS 114 (5), 2017, p. 834 - 839
[4] G. M. Brown et. al., US Patent US 2012/0082904 A1, 2012
[5] K. V. Kravchyk, Chem. Mater. (29), 2017, p. 4484 – 4492
back
Dr.-Ing. Jean-François Drillet
Tel.: 069 / 75 64-476
E-Mail: drillet
M. Sc. Martin Eckert
Tel.: 069 / 75 64-447
E-Mail: eckert
M. Eckert, W. Peters, J.-F. Drillet Materials, 11/12 (2018)
M. Eckert, H. Suthar, J.-F. Drillet Materials, 15/7 (2022)